Have you ever wondered whether Lysol, a staple in many households, is acidic or basic? Understanding the nature of cleaning products like Lysol can make a huge difference in how you use them and the results you get.
Whether you’re a cleaning enthusiast or just trying to keep your home germ-free, knowing this little detail can impact your cleaning routine significantly. Imagine being able to clean more effectively and safely just by understanding the science behind your cleaner.
Curious about how this simple knowledge can transform your cleaning game? Read on to uncover the truth about Lysol’s pH level and how it can affect your home.
Credit: www.chegg.com
Chemical Composition Of Lysol
Have you ever wondered what makes Lysol such an effective cleaning agent? Understanding its chemical composition can help you use it more efficiently in your home. Let’s dive into the active ingredients, the role of surfactants, and the importance of pH balance in Lysol.
Active Ingredients
Lysol contains powerful active ingredients that target germs and bacteria. One key component is benzalkonium chloride, a potent disinfectant. This ingredient breaks down the cell walls of bacteria, effectively killing them.
Another vital ingredient is ethanol, which is known for its antiseptic properties. Ethanol helps dissolve dirt and grease, making surfaces cleaner and safer. These ingredients work together to provide a comprehensive cleaning solution.
Role Of Surfactants
Surfactants play a crucial role in Lysol’s cleaning power. They help the active ingredients spread evenly across surfaces. Surfactants reduce surface tension, allowing the cleaning solution to penetrate grime and dirt.
Think of surfactants as the team players that support the star ingredients. They make sure the cleaning agents reach every nook and cranny. Without surfactants, Lysol wouldn’t be as effective in tackling stubborn stains and residue.
Ph Balance In Cleaning Products
pH balance is vital in cleaning products like Lysol. It determines whether a solution is acidic or basic. Lysol is formulated to be slightly acidic, which enhances its disinfecting capabilities.
Acidity helps break down organic matter and neutralizes bacteria. Have you ever noticed how Lysol leaves surfaces feeling fresh and clean? That’s the result of its balanced pH. A well-balanced pH ensures maximum effectiveness without damaging surfaces.
Next time you grab a bottle of Lysol, consider its chemical composition. How might understanding its ingredients change the way you clean your home?

Credit: www.rbnainfo.com
Acidic Vs Basic Properties
Lysol products are typically acidic. This helps in breaking down bacteria and viruses effectively. Acidic properties enhance cleaning power, especially against tough stains and grime. Understanding the acidic nature aids in choosing the right cleaning solution for your needs.
Understanding the acidic or basic nature of cleaning products like Lysol can make a big difference in how you tackle household chores. The pH level of a cleaner determines its acidic or basic properties, influencing its effectiveness on various surfaces. Knowing whether Lysol is acidic or basic can help you choose the right cleaner for your specific needs and ensure better cleaning results.Understanding Acidity And Basicity
Acidity and basicity are measured on the pH scale, which ranges from 0 to 14. A pH less than 7 indicates acidity, while a pH greater than 7 indicates basicity. Lysol products often contain ingredients like citric acid, making them slightly acidic, which is great for breaking down grime and killing bacteria.Using an acidic cleaner like Lysol can be effective on bathroom surfaces where soap scum and hard water stains are common. However, you should be careful on delicate surfaces, as acidity can sometimes cause damage. Knowing the pH of your cleaning product can help you avoid mishaps and get the most out of your cleaning routine.Impact On Cleaning Efficiency
The acidic nature of Lysol products can enhance their cleaning efficiency, especially against mineral deposits and organic residues. This is because acids are good at dissolving these types of grime.When you use Lysol in your bathroom or kitchen, you’re leveraging its acidic properties to cut through tough stains. But what about those stubborn grease stains in the garage? A basic cleaner might be more effective there, as basic substances excel in breaking down oils and fats.So, you see, knowing the acidic or basic nature of your cleaning product can direct you to the right choice for various tasks. Have you ever wondered why some cleaners work wonders on certain stains but not others?Safety Considerations
While the effectiveness of acidic cleaners like Lysol is undeniable, safety should always be a priority. Using acidic products requires careful handling to prevent skin irritation or damage to surfaces.Always read the label and follow instructions to ensure safe use. Wearing gloves and ensuring proper ventilation are small steps that can make a big difference in safety.You might have had that unpleasant experience of a cleaner leaving an unexpected mark or smell. Understanding the product’s properties can help prevent such surprises. Are you taking the necessary precautions with your cleaning routine?Testing Lysol’s Ph Level
Testing the pH level of Lysol can provide valuable insights into its cleaning properties. The pH scale ranges from 0 to 14, with numbers below 7 indicating acidity and numbers above 7 indicating alkalinity. Understanding where Lysol falls on this scale can help you determine its suitability for different cleaning tasks. Is Lysol acidic or basic? Let’s dive into the testing methods to find out.
Methods For Ph Testing
To test Lysol’s pH level, you can use a simple pH test strip or a digital pH meter. Both methods are straightforward and reliable.
Start by preparing a small sample of Lysol solution mixed with water. Dip the pH test strip into the solution, or insert the pH meter probe.
Read the results immediately. If you’re using test strips, compare the color change to a pH scale chart. Digital meters will give you a direct numerical reading.
Interpreting Results
Once you’ve obtained the pH reading, it’s time to interpret it. If the pH level is below 7, Lysol is acidic. If above 7, it’s basic.
An acidic cleaner can be effective for removing mineral deposits and soap scum. A basic cleaner is great for cutting through grease and grime.
Consider what you’re cleaning. Is it a bathroom sink or a kitchen counter? Knowing the pH helps you pick the right cleaner for the job.
Comparative Analysis With Other Cleaners
How does Lysol stack up against other popular cleaners? Many household cleaners range from mildly acidic to slightly basic.
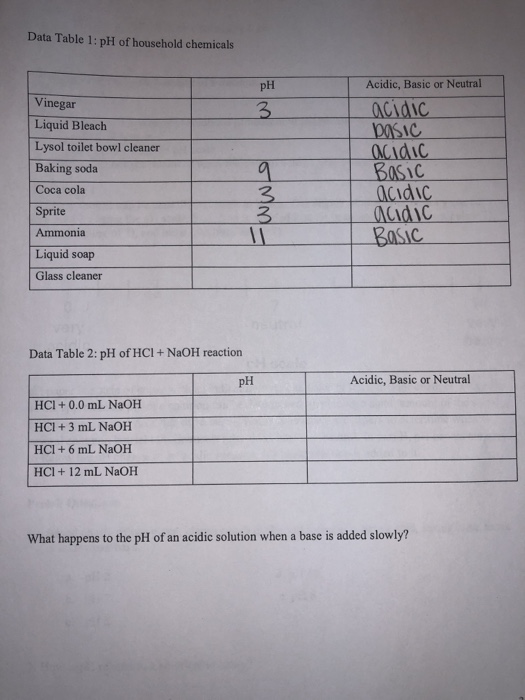
For example, vinegar, a common household cleaner, is acidic with a pH around 2.5, while baking soda is basic with a pH around 9.
Comparing Lysol’s pH level with these can help you understand its cleaning power and versatility. Do you prefer something more acidic or basic for your cleaning needs?
Knowing the pH level of Lysol allows you to make informed decisions. Test it, interpret the results, and compare it with other cleaners to optimize your cleaning routine.
Credit: www.lysol.com
Effects Of Ph On Different Surfaces
Lysol products play a significant role in cleaning and disinfecting. Understanding the effects of pH on different surfaces is crucial. pH levels determine how cleaning agents interact with materials. Surfaces react differently to acidic or basic solutions. This knowledge helps in avoiding damage and ensuring effective cleaning.
Compatibility With Household Materials
Lysol’s pH affects its compatibility with various materials. Acidic cleaners can tackle tough stains but may harm delicate surfaces. Basic solutions are generally gentler but might not remove stubborn grime. Knowing your surface type is important. Wood, marble, and fabric require special attention. Check product labels for material-specific guidance.
Potential Damage Risks
Improper use can lead to damage. Acidic solutions may etch or discolor sensitive surfaces. Basic cleaners might cause fading over time. Porous materials are especially vulnerable. Test a small area first. Observe any adverse reactions. Adjust cleaning methods accordingly to prevent costly repairs.
Best Practices For Use
Follow best practices to ensure safe cleaning. Dilute solutions as recommended. Use protective gloves when handling strong cleaners. Ventilate areas to avoid inhaling fumes. Apply Lysol with a soft cloth or sponge. Rinse thoroughly after cleaning. Regular maintenance prevents buildup and preserves material integrity.
Environmental Impact
Exploring the pH level of Lysol reveals its environmental impact. Lysol products typically have a pH level that can be acidic or basic, affecting their interaction with surfaces and the environment. Understanding its composition helps consumers make informed choices for eco-friendly cleaning.
When discussing the environmental impact of cleaning products like Lysol, it’s essential to consider their chemical composition. Is Lysol acidic or basic? This question not only affects its cleaning efficacy but also its impact on the environment. Understanding this can help you make more informed decisions about the products you use daily.Biodegradability Concerns
Many household cleaning products contain chemicals that may not break down easily in the environment. Lysol, with its active ingredients, poses similar concerns. Are these chemicals capable of decomposing without leaving harmful residues?A significant factor to consider is how long these chemicals linger in the environment. You might wonder if the convenience of a quick spray is worth potential long-term harm. Opting for products with known biodegradable ingredients can lessen this impact.Influence On Water Sources
The chemicals in Lysol can enter water sources through various pathways. Think of the residue that rinses down your sink or toilet. What happens once these substances reach rivers or lakes?These chemicals may affect aquatic life and disrupt ecosystems. You could reduce this impact by using less product or ensuring proper disposal. Have you considered how your cleaning habits might contribute to water pollution?Regulations And Standards
There are standards in place that regulate the environmental impact of household cleaners. These rules ensure that products like Lysol meet certain safety and ecological criteria. But are these regulations stringent enough to protect our environment?Manufacturers must adhere to specific guidelines, but consumer awareness is key. Are you familiar with the labels and certifications that indicate a product’s environmental compliance? Choosing products that meet high environmental standards can make a difference in your ecological footprint.Alternatives To Lysol
Many people search for alternatives to Lysol due to its chemical nature. Some prefer eco-friendly options or DIY solutions. Others compare effectiveness to find the best choice. This section explores these options in depth.
Eco-friendly Options
Eco-friendly cleaners offer a safer choice for the environment. Brands like Seventh Generation and Method use natural ingredients. They avoid harsh chemicals found in traditional cleaners. These products are often biodegradable. Some even use recycled packaging. They aim to clean effectively without harming nature.
Diy Cleaning Solutions
DIY cleaning solutions are easy to make at home. Common ingredients include vinegar, baking soda, and lemon juice. Vinegar is acidic and can cut through grime. Baking soda acts as a gentle scrub. Lemon juice adds a fresh scent and has antibacterial properties. These ingredients are often found in most kitchens. They offer a cost-effective way to maintain a clean home.
Comparing Effectiveness
Effectiveness varies between Lysol, eco-friendly, and DIY options. Lysol is known for its strong disinfecting power. It kills germs quickly and efficiently. Eco-friendly cleaners are milder but still effective. They work best on everyday dirt and stains. DIY solutions are great for light cleaning tasks. They may not be as powerful as commercial products. Each option has its pros and cons depending on needs.
Frequently Asked Questions
Is Lysol Acidic Or Basic?
Lysol is slightly acidic. It helps kill germs effectively.
Can Lysol Damage Surfaces?
Yes, Lysol can damage some surfaces. Test on a small area first.
What Ph Level Is Lysol Cleaner?
Lysol’s pH is usually around 6. 5. It is mildly acidic.
Is Lysol Safe For Kitchen Use?
Lysol is safe for kitchens. Ensure proper ventilation when using.
How Does Lysol Kill Germs?
Lysol kills germs by disrupting their cell walls. Effective against bacteria and viruses.
Conclusion
Understanding if Lysol is acidic or basic helps in its safe use. Lysol’s composition can influence its cleaning effectiveness and safety. Knowing its pH level aids in choosing the right surface. Always read labels and follow instructions carefully. Using Lysol correctly ensures effective cleaning and germ removal.
Safety is important, so handle with care. Ensure proper ventilation while using. Proper usage keeps your home clean and healthy. Educate yourself on cleaning products for best results. Awareness leads to better cleaning decisions. Stay informed and make safe cleaning choices.
